That's essentially true for most "normal" applications, but even in physics, laws are meant to be broken at some point. He said 1% at 300000 psi. That's a barely noticeable change at 20,000x atmospheric pressure.
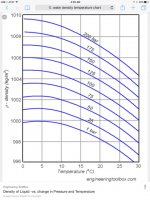
Water is unique because it reaches its smallest volume/highest density at 4 degrees C, so technically it compresses (contracts is prolly a better word) as it warms or cools to that point even without changing pressure. And expands as it moves away from that point. It's a very small amount and if contained, that small amount can exert an astounding amount of pressure. That's why we have expansion joints/tanks on our water heaters. And also why, for the most part, it's considered incompressible.
Once it reaches 0 degrees all hell breaks loose. All this is a good thing, since otherwise our oceans would freeze from the bottom making it much more difficult for life to take hold.
Now you're gonna start calling me Prime9.
:blush:
Dave
View attachment 238180
Sent from my iPad using
WAYALIFE mobile app